Abstract
Despite recent transformative advancements in gene editing tools and hematopoietic stem and progenitor cell (HSPC) gene therapies, there are still unanswered questions about the impact of CRISPR/Cas9 and base editing technologies on HSPC clonal behavior, function, and integrity in vivo. Better understanding of the impact of available gene therapy modalities on HSCs in a relevant animal model should help safely move these therapies forward for clinical use and provide information regarding their relative advantages and risks. CRISPR/Cas9 editing generates double strand DNA breaks (DSBs) at targeted genomic locations and potentially at off-target sites, resulting in potential HSC genotoxicity and loss. To counter DSB-related consequences, base editors (BEs) have been developed with the ability to produce single-nucleotide conversion without DSBs, avoiding potentially deleterious DNA damage repair pathways.
CD33 is expressed on all myeloid cells downstream of the common myeloid progenitor and is used as both a diagnostic marker and a therapeutic target for acute myeloid leukemia. Our past work demonstrated successful Cas9/NHEJ-mediated CD33 KO in rhesus macaque (RM) HSPCs with no functional impact (Kim et al, Cell, 2018). In the current study, we first genetically barcoded RM HSPCs, and then knocked out the CD33 locus with the intent of comparing the clonal impact of CRISPR/Cas9 editing via non-homologous end joining (NHEJ) to base editing in RM HSPCs in vitro by myeloid differentiation assays and in vivo following autologous HSPC transplantation. Highly diverse DNA barcodes were introduced into RM HSPCs via transduction with a lentiviral vector also expressing GFP. 24 hours later, the cells were electroporated with either Cas9/NHEJ or BE complexes targeted to disrupt CD33. Cells were further cultured in myeloid differentiation media for 8 days in vitro for differentiation assays (IVD). Cell viability and proliferation were analyzed over time during IVD. DNA was collected on day 8 for editing efficiency and barcode clonal analysis. In addition, GFP and CD33 expression were analyzed by flow cytometry to assess CD33 KO efficiency and examine whether the addition of gene editors impacted the clonality of the HSPCs as assessed by barcode retrieval, and whether the initial lentiviral transduction impacted editing efficiency. HSPC colony-forming unit (CFU) assays were carried out to assess differentiation potential of the transduced and/or edited HSPCs and check for biallelic versus monoallelic editing status at a clonal level.
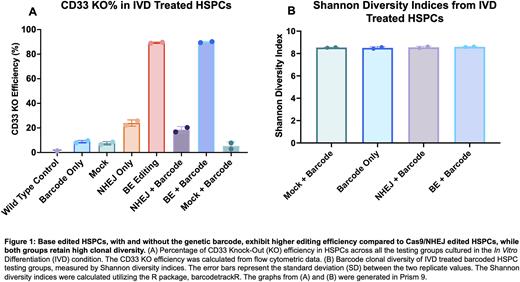
Throughout the 8 days of IVD, cell numbers increased overtime and maintained high cell viability (80-95%) in all test groups, which indicates barcoded lentiviral transduction and gene editing procedures had minimal impact on cell proliferation and viability. Base editing resulted in 80-85% CD33 KO efficiency, regardless of barcode lentivirus transduction prior to gene editing. However, Cas9/NHEJ was significantly less efficient, with CD33 KO efficiency of 10-20%, requiring additional optimization (Figure 1A). Variant allele frequency analysis confirmed the relative levels of CD33 locus editing by both editing methods. CFU assays indicated the lentiviral transduction and gene editing methods do not alter the HSPC differentiation potential as assessed in vitro. Barcode clonal analysis revealed both NHEJ and BE test groups demonstrated retention of high clonal diversity as assessed by Shannon Diversity Index, all > 8 regardless of gene editing methods (Figure 1B).
In summary, lentiviral barcode transduction and editing procedures had no negative impact on cell viability and proliferation. Base editing allowed very high gene editing efficiency in HSPCs and can serve as a powerful editing tool. Importantly, both editing methods resulted highly diverse polyclonal clonal patterns in vitro long term, with no significant differences compared to the non-edited group. Combining genetic barcode technology and the BE or Cas9/NHEJ editing in an in vivo RM autologous HSPC transplantation model to track the hematopoietic clonal reconstitution of the edited HSPCs over time and compare the impact of BE or Cas9/NHEJ editing on engrafting HSPCs clonal behavior is ongoing. Therefore, these clinically relevant findings can determine which gene editing method is more suitable for developing human HSPC gene therapies.
Disclosures
Liu:Beam Therapeutics: Consultancy, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties; Prime Medicine: Consultancy, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties; Pairwise Plants: Consultancy, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Exo Therapeutics: Consultancy, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Chroma Medicine: Consultancy, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Resonance Medicine: Consultancy, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Tevard Biosciences: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Voyager Therapeutics: Consultancy, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Nvelop Therapeutics: Consultancy, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Insitro: Consultancy, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal